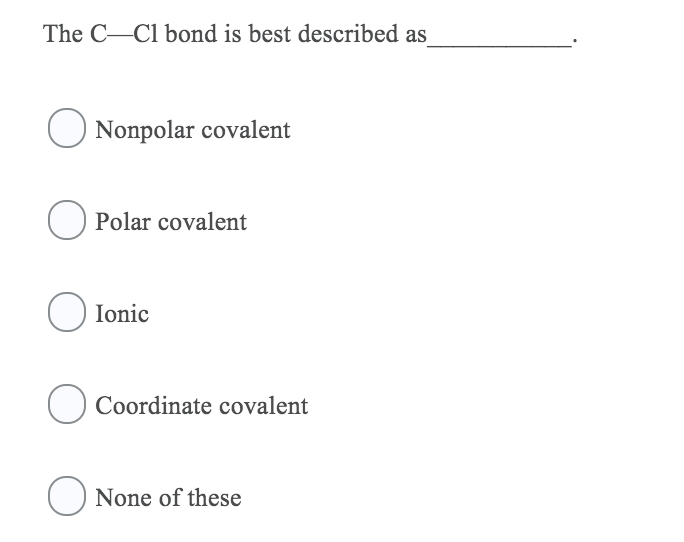
The C Cl Bond Is Best Described As___________
Which one of the following compound is most likely to be an ionic compound. Reaction 1 Reaction 2 BC BC lOOKJ XYXY 4OOKJ.

Solved Your Answer Is Incorrect Try Again The Cl Ci Bond Of Chegg Com
CH3Cl is always polar.

. C Cl 2 and Cl 2. A Two electrons are shared. The asymmetric shape and electronegativity difference between atoms is an important aspect in determining whether a molecule is polar or not.
BIt contains ionic bonds and has a high melting point. The bond energy of each carbon-oxygen bond in carbon dioxide is _____. Repulsion by the valence electrons of the atoms D.
An ionic bond is best described as A the sharing of electrons. The Cl-Cl bond of Chlorine is best described how. Although the four bonds C-Cl are polar because of the difference in electronegativity of Chlorine 316 and Carbon 255 CCl4 is nonpolar because the bond polarity gets canceled with each other due to the symmetrical geometrical structure tetrahedral of the CCl4 molecule.
What statement best describes the polarity of CH3Cl. E the attraction between two metal atoms. The C-Cl bond is best described as Nonpolar covalent Polar covalent Ionic O Coordinate covalent O None of these Identify the relationship between the following two structures.
Write a Lewis structure for the chlorate ion ClO. The C-Cl bond has a large difference in electronegativity compared to the H-C bonds. Reaction CH4 4Cl2.
22Which statement best describes the substance that results when electrons are transferred from a metal to a nonmetal. The bond fission that leads to the production of excited-state radicals with a low kinetic energy release has an angular distribution best described by a negative anisotropy parameter. What statement best describes the polarity of CH3Cl.
C the attraction that holds the atoms together in a polyatomic ion. 1 point a 368 kJ b 1472 kJ c 736 kJ d 2944 kJ 2-Use the table to answer the question. Hence option A is correct.
6 Given the reaction. The very different angular distributions suggest that two different excited states of methyl chloroformate lead to the formation of ground- and excited-state. A chemical bond between two atoms results from a simultaneous A.
When a bond if formed energy is ReleasJ When a bond is broken energy i A b So rb e-d The more energv that is given off during the formation of a bond the hno g stable the bond and themore the compound. Repulsion by the protons in the two nuclei 32. H 2 Cl 2 2HCl Which of the following statements best describes in this reaction.
8 at carbon and S-at chlorine B ionic C nonpolar. TRUEFALSE The bond in F 2 is described as polar covalent. B Polar covalent.
Cl Mg 68. Hence double bond character in carbon chlorine bond is maximum and bond length is shortest. Orbitals that are equivalent in energy are referred to as.
The C-Cl bond has a large difference in electronegativity compared to the H-C bonds. The C-Cl bond is best described as A. Attraction by the protons for the neutrons B.
D Four electrons are transferred. Between 100C and 900C. Bonds are forces that hold 2 atoms together in a compound.
No dipole D polar. Al K c. Which formula represents a mo ecu ar compound.
Is more stable than resonance form of any other given compounds. Molecule is best described as A linear. DIt contains covalent bonds and has a high melting point.
Induction and Polar Covalent bond Section. ОН ОН ОН В. For detailed information you must read out an article on the polarity of CH2Cl2.
OH OH Enantiomers Identical Neither Which of the following is the correct structure for the compound R-2-pentanol. In turn this carbon atom drags electron density partially from the next carbon which also gets partial positive charge. This molecule is best described as 1 nonpolar with nonpolar covalent bonds 2 polar with nonpolar covalent bonds 3 nonpolar with polar covalent bonds 4 polar with polar covalent bonds ___ 27 Which compound is ionic.
2CO O2 2CO2 Carbon monoxide and oxygen combine to produce carbon dioxide. Thus the first carbon atom gets partial positive charge. The C Cl bond is best described as___________.
A Nonpolar covalent B Polar covalent C Ionic D Coordinate covalent E None of these Ans. The Cl Cl bond is best described as A nonpolar covalent B polar covalent C ionic from CHM 241 at Northern Virginia Community College. Four electrons are shared.
Carbon Tetrachloride is an IUPAC. Contains only ionic bonds solid A contains only ionic bonds and solid B contains only covalent bonds 5. The C-Cl bond in the butyl chloride CH 3 -CH 2 -CH 2 -CH 2 -Cl is polarized due to electronegativity difference.
Bond Energy Released in Formation kcalmole. The total bond energy of the products is 1472 kJ. Attraction by the two nuclei for the electrons C.
CH3Cl is a fairly polar molecule. Which of the following statements best describes the C-Cl bond in the following compound. Resonance form ClCHCHNO 2.
CH3Cl is a fairly polar molecule. 1 breaking HH bonds releases energy 2 forming HCl bonds absorbs energy. 50 the c cl bond is best described as a nonpolar.
How many distinct p orbitals exist in the 2nd electron shell. Solve any question of Chemical Bonding and Molecular Structure with-. CIt contains covalent bonds and has a low melting point.
The electron configuration of a carbon atom has how many electrons unpaired. Thus C-Cl bond is polar and the overall charge distribution across the molecule is non-uniform. D the attraction between two nonmetal atoms.
AIt contains ionic bonds and has a low melting point. C Two electrons are transferred. According to the VSEPR theory the molecular geometry of ammonia is.
Bonds that have an even distribution of charge. 1 N2O 2 SO 2 3 CaCl 2 4 HCl ___ 28 What type of bonding is found in the molecule HBr. CCl4 Carbon tetrachloride is nonpolar in nature.
1- Use the reaction to complete the sentence. D LiCl C KCI 6. The electrons are withdrawn by the chlorine atom.
-at carbon and 8 at chlorine E None of these. Which statement describes a multiple covalent bond. Which of the following statements best describes the C-Cl bond in the following compound.
C S d. HH cic C- H HHHH A polar. Which statement best describes how a catalyst can speed up a chemical reaction.
1 metallic 2 nonpolar covalent 3. 4 The water loses heat and the block gains heat until both are at the same temperature between 100C and 900C. B the transfer of electrons from one atom to another.
CH3Cl is always polar.
Solved The C Cl Bond Is Best Described As Nonpolar Covalent Chegg Com
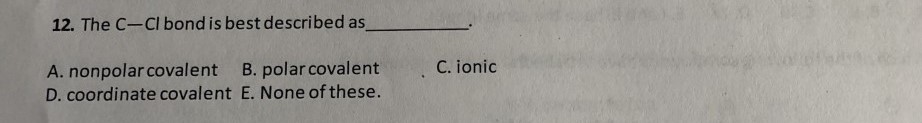
Answered 12 The C Cl Bond Is Best Described As Bartleby
Solved The C Cl Bond Is Best Described As Nonpolar Covalent Chegg Com
No comments for "The C Cl Bond Is Best Described As___________"
Post a Comment